These attractive forces are due to electrostatic forces. These attractive forces are due to electrostatic forces.
Khan Academy offers practice exercises instructional videos and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom.
Surface tension khan academy. Test your knowledge on cohesion adhesion and surface tension of water. If youre seeing this message it means were having trouble loading external resources on our website. David explains the concepts of surface tension cohesion and adhesionWatch the next lesson.
Ram explains the concepts of surface tension cohesion and adhesion. If youre seeing this message it means were having trouble loading external resources on our website. Surface tension in water and how the surface tension is related to hydrogen bondingWatch the next lesson.
Cohesive forces are responsible for surface tension a phenomenon that results in the tendency of a liquids surface to resist rupture when placed under tension or stress. Water molecules at the surface at the water-air interface will form hydrogen bonds with their. Khan Academy offers practice exercises instructional videos and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom.
We tackle math science computer programming history art history economics and more. Surface tension Water acids and bases. Ram explains the concepts of surface tension cohesion and adhesion.
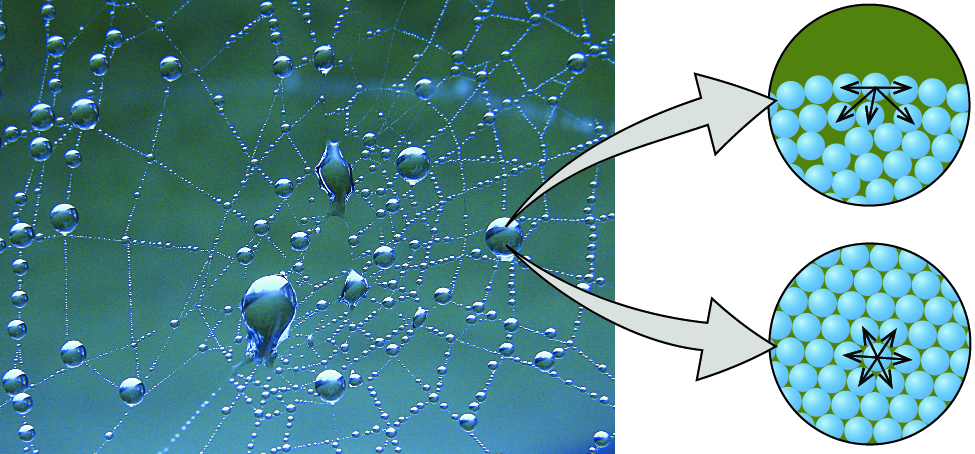
Only water has an unusually high surface tension because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds water has a higher surface tension. Surface tension is the attraction that happens between water molecules. Water molecules are attracted to each other.
The surface of water has an elastic quality because the molecules are hugging close together. This is why some insects can walk on water. How capillary action and the meniscus are related to intermolecular forces in water.
Watch the next lesson. Surface tension is a contractive tendency of the surface of a liquid that allows it to resist an external force. Attractive forces between molecules of the same type are called cohesive forces.
Liquids can for example be held in open containers because. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid minimizing the surface area. These attractive forces are due to electrostatic forces.
Surface tension is defined as the energy required to increase the surface area of a liquid or the force required to increase the length of a liquid surface by a given amount. This property results from the cohesive forces between molecules at the surface of a liquid and it causes the surface of a liquid to behave like a stretched rubber membrane. Ctrl click Double click.