UNLOCK FULL SOLUTION 197. This conversation has been flagged as incorrect.
Solid solutions are only formed with metals.
Solid solutions are only formed with metals. Solid solutions are only formed with metals. True False See answers 1 Ask for details. Follow Report Log in to add a comment to add a comment.
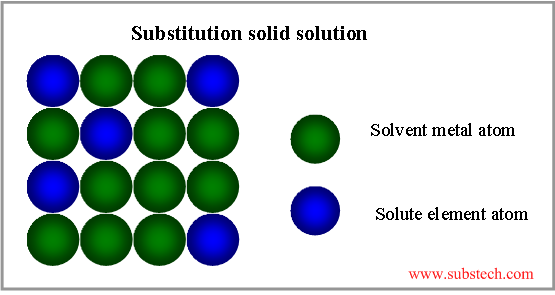
When the liquid solution of two metals crystallises and if a solid of only single crystal structure forms then a solid solution has formed. This happens when atoms of two metals are able to share together a given crystal structure normally of the solvent metal such that even in a unit cell of this crystalline solid both type of atoms are present in proportion to their concentration Fig. Thus a solid solution.
Solid solutions are only formed with metals. Log in for more information. Asked 10102012 93231 AM.
Updated 4162018 121300 PM. This conversation has been flagged as incorrect. Solid solutions are only formed with metals.
Log in for more information. Added 4162018 121259 PM. This answer has been.
Solid solution is a phase where two or more elements are completely soluble in each other. Depending on the ratio of the solvent matrix metal atom size and solute element atom size two types of solid solutions may be formed. Solid solutions of introduction - are formed when atoms or ions of one substance are embedded or placed between the atoms or ions of another substance.
Properties of solid solutions. Properties of solid solutions differ significantly from the properties of the components. Solid solutions are technically more valuable than the pure components.
They have higher hardness lower electrical conductivity than the metals - their components. In solid solutions have solvent and solute. Solid solution A solid solution is formed when two metals which are mutually soluble in the liquid state remain dissolved in each other after crystallization.
If the resulting structure is examined even with the aid of a high power electron microscope no trace can be seen of the parent. 2311 Solid Solutions A solid solution is formed by mixing a foreign element B called an impurity or solute with a perfect crystalline element A called the host or solvent such that the atoms of B share the various crystal sites of element A. Solid solution mixture of two crystalline solids that coexist as a new crystalline solid or crystal lattice.
The mixing can be accomplished by combining the two solids when they have been melted into liquids at high temperatures and then cooling the result to form the new solid or by depositing vapours of the starting materials onto substrates to form thin films. In order for one type of atom to substitute for another in a solid solution the substituting atom must be. Similar in size and from the same group is it likely that an atom of Te would substitute for an atom of O in a solid compound.
A solid solution occurs when we alloy two metals and they are completely soluble in each other. If a solid solution alloy is viewed under a microscope only one type of crystal can be seen just like a pure metal. Solid solution alloys have similar properties to pure metals but with greater strength but are not as good as electrical conductors.
Phases in Metals Solid solution - When in a solid the atoms of solute are present in the lattice of the solvent it is known as solid solution. Intermetallic compound a compound formed by two or more metals such as AlLi. Interstitial solid solutions are solid state solutions that form when solute atoms enter into the holes between solvent atoms of lattice.
These solid solutions form only if the solute atoms are small enough to enter the holes of the lattice. In addition the atomic size of solute atoms should be about 40 of the size of solvent atoms in order to form this type of lattice. This is the main difference between.
Solid solutions of metals or nonmetals dissolved in metals are. Solution Particles of two or more substances that are distributed evenly among each other an. UNLOCK FULL SOLUTION 197.
Get solution in 10 Seconds. Atomic radius crystal structure electronegativity and the most common valence are given in the following table for several elements. For those that are nonmetals only atomic radii are indicated.