Adjusting soil pH is not an exact science and takes time. For example increasing the amount of sodium in an alkaline soil tends to induce dissolution of calcium carbonate which increases the pH.
Vinegar is a diluted liquid form of acetic acid and depending on what the vinegar is made from and how its processed it may also contain other things like traces of vitamins and minerals.
Soil ph adjustment calculator. Clemson Soil Acidification Calculator. For most South Carolina soils and crops soil acidification is not necessary. In some instances however lowering the pH is desirable.
Adjusting soil pH is not an exact science and takes time. A pH test number that is more than 05 on either side of the optimal pH number for the plants you want to grow will require a soil amendment or additive to adjust the pH. A pH test number that is within 05 of the optimal pH number for the plants you want to grow does not require soil amendments.
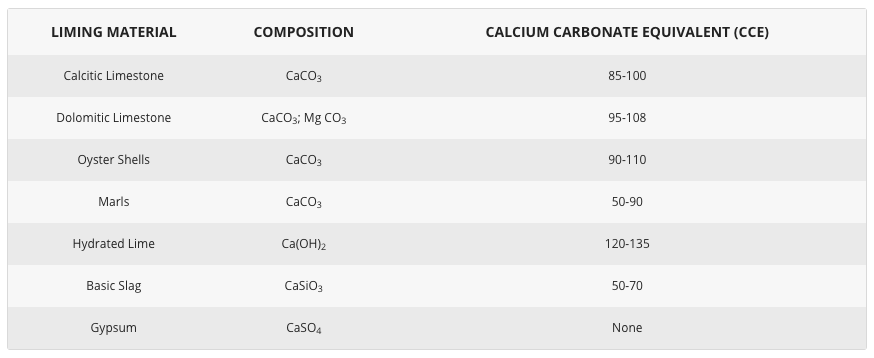
The quickest way to change pH is to add. Calculate the proper amount of lime to apply for your Clemson soil test results and target pH. View all Clemson Precision Ag Calculators.
Clemson Lime Rate Calculator. The agricultural limestone rates calculated here are only applicable to results from the Clemson University Soil Testing Laboratory. For calculating rates for a.
The Lime Calculator allows users to calculate a recommended liming rate tha based on the cropping enterprise type measured soil pH soil type and liming material to be applied. The system uses ALA recommendations and was developed in partnership with The University of Hertfordshire. Agriculture and the Environment Research Unit AERU.
Select Farming Enterprise Arable Grass. Add a few drops of vinegar to a tablespoon of dry garden soil. If it fizzes your soils p H is greater than 75.
Add a pinch of baking soda to a tablespoon of moist soil. If it fizzes your soils p H is less than 50. Correcting Soil pH What Is Soil pH.
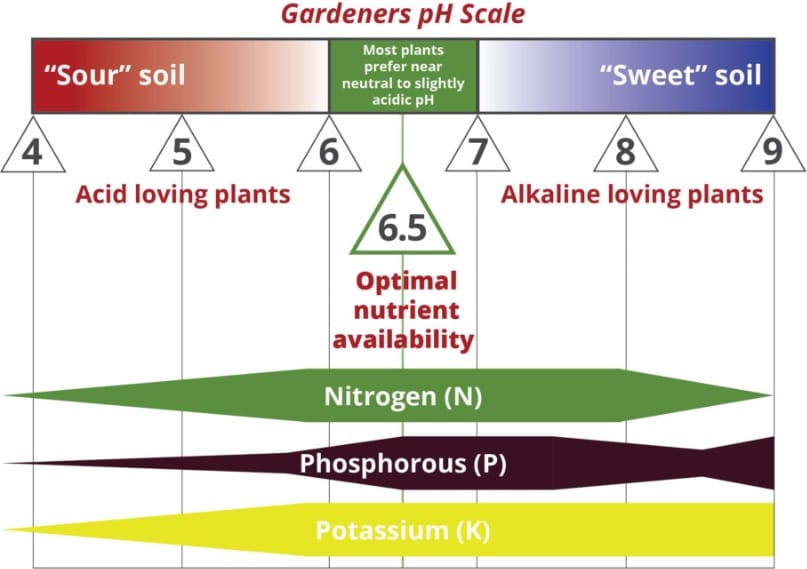
The term pH refers to the acidity or alkalinity of the soil. A pH of 70 is neutral while that above 70 is alkaline sweet or basic. Soil with a pH of 69 or below is acidic or sour.
Why Is the Soil pH Important. The degree of acidity or alkalinity of a soil will determine whether nutrients may be absorbed by plants from the soil. For instance as soil.
Allow the probe to sit in the muddy water for about a minute before taking it for pH reading. A pH value above 7 indicates an alkaline soil while a pH value under 7 indicates acidic soil. Accurate reading is all about empirical analysis from different spots.
AlkCalc - This calculator provides the recommendations for the amount of acid to add to irrigation water in order to modify the pH and alkalinity levels. It also provides the amount of added phosphorus nitrogen and sulfur that the corresponding acids will provide plus an economic comparison of each acid. Check the new pH and calculate the change in pH.
Now that you know how much the pH changes for a particular solenoid actuation time calculate how long the solenoid valve needs to. With this pH calculator you can determine the pH of a solution in a few ways. It can convert pH to H as well as calculate pH from the ionization constant and concentration.
PH is an essential factor in chemistry medicine and daily lifeRead the text below to find out what is the pH scale and the pH formulaIn the end we will also explain how to calculate pH with an easy step-by-step. I want to adjust the certain amount of soil pH precisely. How much CaCO3 I should add into 200 g soil to increase the pH from 4 to 8.
And would this change other properties of soil eg. In order to adjust your soils pH you will need to know what that is. A soils pH represents how acidic or alkaline it is.
Soil pH is determined on a scale from zero to 14 with seven being a neutral pH. This potent liquid is also useful to gardeners and it can be used to naturally adjust the pH level of soil without the need for harsh commercially manufactured products. Vinegar is a diluted liquid form of acetic acid and depending on what the vinegar is made from and how its processed it may also contain other things like traces of vitamins and minerals.
The average pH of. Managing soil pH is essential to creating ideal growth conditions for most plants. This is because the pH of the soil controls the solubility of nutrients as well as toxic metals.
Because of this most plants have a preferred range in soil pH. Under most cases liming agents are added to soil to raise pH to the desired range. However in some cases lower soil pH is desired which can be.
Soil pH adjustments with elemental sulfur should be monitored over time with routine soil sampling and analysis. This will ensure that the sulfur applied is having the desired effect on soil pH. Soils that are overacidified due to sulfur application soil pH is lower than desired should be limed to neutralize soil pH to the desired soil pH level.
Soils that are underacidified soil pH is. This calculator attempts to correct the reading of a pH meter that does not internally account for temperature. If your pH meter features automatic temperature compensation ATC or the ability to dial-in temperature it is already internally performing this correction.
Remote sensing of vegetation is a useful tool for many applications in ecology agriculture and beyond. This short tutorial showing you how to calculate the. For example increasing the amount of sodium in an alkaline soil tends to induce dissolution of calcium carbonate which increases the pH.
Calcareous soils may vary in pH from 70 to 95 depending on the degree to which Ca2.