Not that how much thermal energy the system has. This energy enters the cold body at a lower temperature.
Thats the energy that makes molecules in a fluid move randomly.
Similarities between thermal energy and temperature. Thermal energy is energy. That is the energy that is transferred from one system to another without mechanical means. Thats the energy that makes molecules in a fluid move randomly.
Thats the kind off energy one uses to run a Carnots engine. It is the measure of how hot a system is. Not that how much thermal energy the system has.
There can be two systems with same temperature but different thermal energies. Temperature is a measure of average kinetic energy per molecule in a substance while heat is the form of energy transferred between two materials that are at different temperatures. Heat flows between materials from higher temperature to lower temperature until thermal.
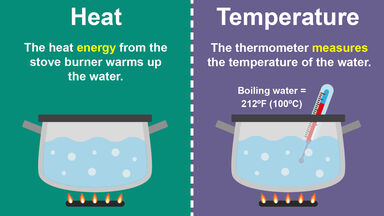
Temperature is more concerned with molecular kinetic energy. Temperature describes the average kinetic energy of molecules. HEAT deals with thermal energy.
Thermal energy can be otherwise understood as the total microscopic kinetic and potential energy of a system. So we can say that heat is afunction of Temperatureenergy stored in molecules. Difference Between Thermal Energy and Temperature Thermal energy is not a directly measurable quantity whereas temperature is a measurable quantity.
The temperature of an object can take negative values depending on the unit system used to measure the temperature. Temperature is measured in. Main Differences Between Temperature and Thermal Energy The average kinetic energy of molecules within an object is called temperature.
Whereas the total kinetic energy within. For temperature the state can vary it can either be hot or cold but in the case of thermal energy the temperature of. This means that the same amount of energy is transferred to both.
However the smaller volume of water increases in temperature more. The same amount of energy has been transferred to a smaller. Thermal energy includes all the kinetic energy of molecules in an object while the temperature is a physical property that measures the average kinetic speed of molecules.
The thermal energy measures the mass of a substance. The temperature of the hot body falls. This energy enters the cold body at a lower temperature.
The cold body gains energy and its temperature rises. The transfer of energy continues till both the bodies have the same temperature. The form of energy that is transferred from the hot body to a cold body is called heat.
Thus Heat is the energy that is transferred from one body to the other in thermal. It is the thermal energy. Temperature is not an energy.
It is the thermal state of a physical body or thermodynamic system. In classical mechanics temperature of a body indicates the average kinetic energy of all the molecules of the corresponding body. Heat flow is a.
Temperature is a parameter which can be directly measured with the help of various devices such as thermometers whereas thermal energy can be calculated. For example nobody can predict the amount of thermal energy in a black box using a thermometer. The main difference is that heat deals with thermal energy while temperature is more concerned with molecular kinetic energy.
As against temperature is the measure of the amount of thermal energy. This is the reason we have already mentioned in the beginning itself that the two are related to each other. There exists a direct relationship between heat and temperature.
This means when the heat content is higher then the temperature will be more. A piece of iron is placed in a kiln until it reaches the temperature θ of the kiln. The iron is then quickly transferred to water held in a thermally insulated container.
The water is stirred until it reaches a steady temperature. The following data are available. Thermal capacity of the piece of iron 60JK 1.
The core differenceis that heat deals with thermal energy whereas temperatureis more concerned with molecular kinetic energy. Heat is the transfer of thermal energy whereas temperatureis a property the object exhibits. Click to see full answer.
Then how is temperature different from thermal energy.