These are stereoisomers in which the functional groups position is rotated around the alkene bond. Maleic acid is the cis isomer and fumaric acid is the trans isomer.

Geometric or cis-trans isomers of alkenes including halogenated alkenes tutorial with worked examples for chemistry students.
Examples of cis and trans isomers. Maleic acid is the cis isomer and fumaric acid is the trans isomer. Elaidic acid and oleic acid are cis-trans isomers. The former is solid at room temperature melting point 43 o C and the latter is found to be liquid with a melting point of 134 o.
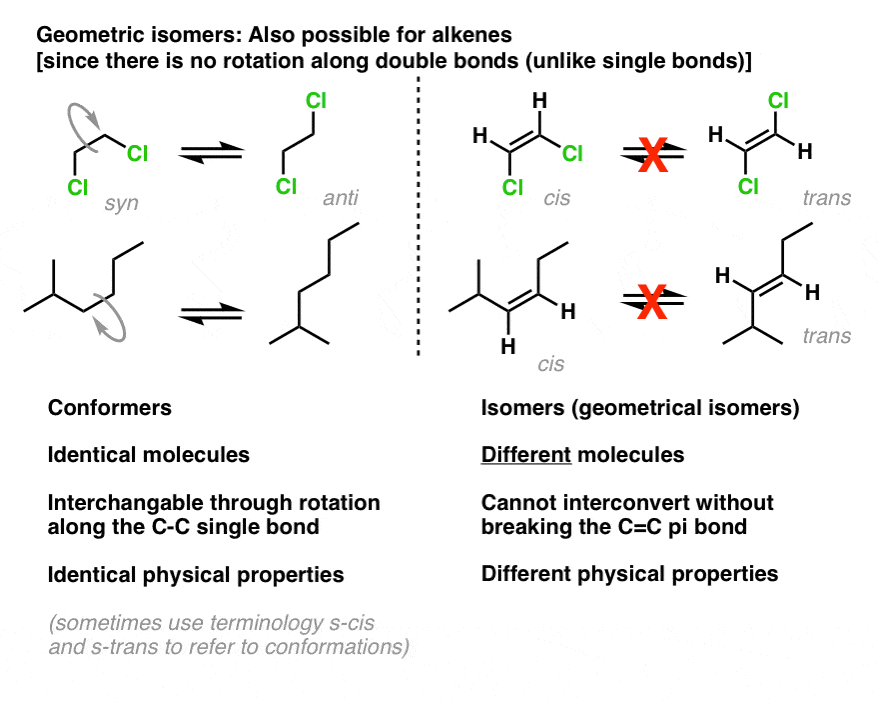
The isomer in which the two chlorine Cl atoms lie on the same side of the molecule is called the cis isomer Latin cis meaning on this side and is named cis-12-dichloroethene. The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomer Latin trans meaning across and is named trans -12-dichloroethene. Cis Trans Isomers Examples.
Cis and trans isomerism exist in both organic and inorganic compounds. These are stereoisomers in which the functional groups position is rotated around the alkene bond. Depending upon these functional groups positions around the carbon-carbon double bond the molecules with the same number of atoms of each element are named differently.
12-dichlorethene In the example on the left the chlorine atoms can be opposite or across from each other in which case it is called the trans isomer. If the the chlorine atoms are next to or adjacent each other the isomer is called cis. One isomer has substituents on the same side cis of the double bond.
The other has substituents on the opposite side trans. B Cyclic compounds Cyclic compounds can exist as cis-trans because there is restricted rotation about single bonds. For example consider 12-dimethylcyclohexane.
In geometrical isomer nomenclature the prefix cis- and trans- are used to identify which side of the double bond the similar atoms are found. The cis- prefix is from the Latin meaning on this side. In this case the chlorine atoms are on the same side of the carbon-carbon double bond.
This isomer is called cis-12-dichloroethene. Usually cis and trans isomerism can be found in organic and inorganic compounds alkanes and in alkenes. Cis isomers are isomers in which two similar atoms lie on the same side of a double bond in the molecule.
On the other hand trans isomers feature molecules with two similar atoms placed on opposite sides of a double bond. Cis-trans isomerism describes the difference between molecules having the same connectivity of atoms but different properties. These different properties are caused due to the difference in the spatial arrangement of the two molecules.
The main difference between cis and trans isomers is that cis isomers are essentially polar whereas trans. Give one example using a 4C alkene with a halogen substituent. Determine the product of a hydration reaction on 1-hexene and draw the reaction.
Explain cis trans isomers and in particular why they only occur in alkenes and not alkanes. Give one example using a 4C alkene with a halogen substituent. Trans isomers differ from cis isomers in more than just appearance.
Physical properties also are affected by conformation. For example trans isomers tend to have lower melting points and boiling points than corresponding cis isomers. They also tend to be less dense.
Geometric or cis-trans isomers of alkenes including halogenated alkenes tutorial with worked examples for chemistry students. So all 4 of the structures we have drawn are actually exactly the same. 112-dibromoeth e ne does NOT display cis-trans isomerismThis problem has been solved.
See the answerIn a complex such as CoCl3и4NH3 the. Example alkenes 2 butene exists has two cis trans isomers C C H 3 C H CH3 H C C from CHM 258 at University Teknology Mara Campus Arau Perlis - Malaysia. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers.
Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal gas constant 1. There must be stronger intermolecular forces between the molecules of the cis isomers than between trans isomers. Taking 12-dichloroetheneas an example.
Both of the isomers have exactly the same atoms joined up in exactly the same order. That means that the van der Waals dispersion forces between the molecules will be identical in both cases. This organic chemistry video tutorial provides a basic introduction into cis and trans isomers using alkenes and cycloalkanesSubscribehttpswwwyoutubec.
Examples of this are the oleic acid cis isomer and elaidic acid trans isomer. The cis isomers melting point is 134 C the trans isomer melts at 43 C. The reason for this is that the trans isomer is straighter packs better and hence having a much higher melting point.
Other kinds of cis and trans isomers exist in ring compounds. For example cis - and trans -12-dimethylcyclopropane are stereoisomers. In the figure below bp stands for boiling point This time there is no imaginable rotation about bonds that can equilibrate the two isomers so these two molecules are not conformational isomers.